What is a Microreactor?
A microreactor is a compact nuclear reactor designed to generate up to 20 megawatts of thermal energy. It's revolutionizing energy production by offering portable, safe, and efficient power solutions for various applications.
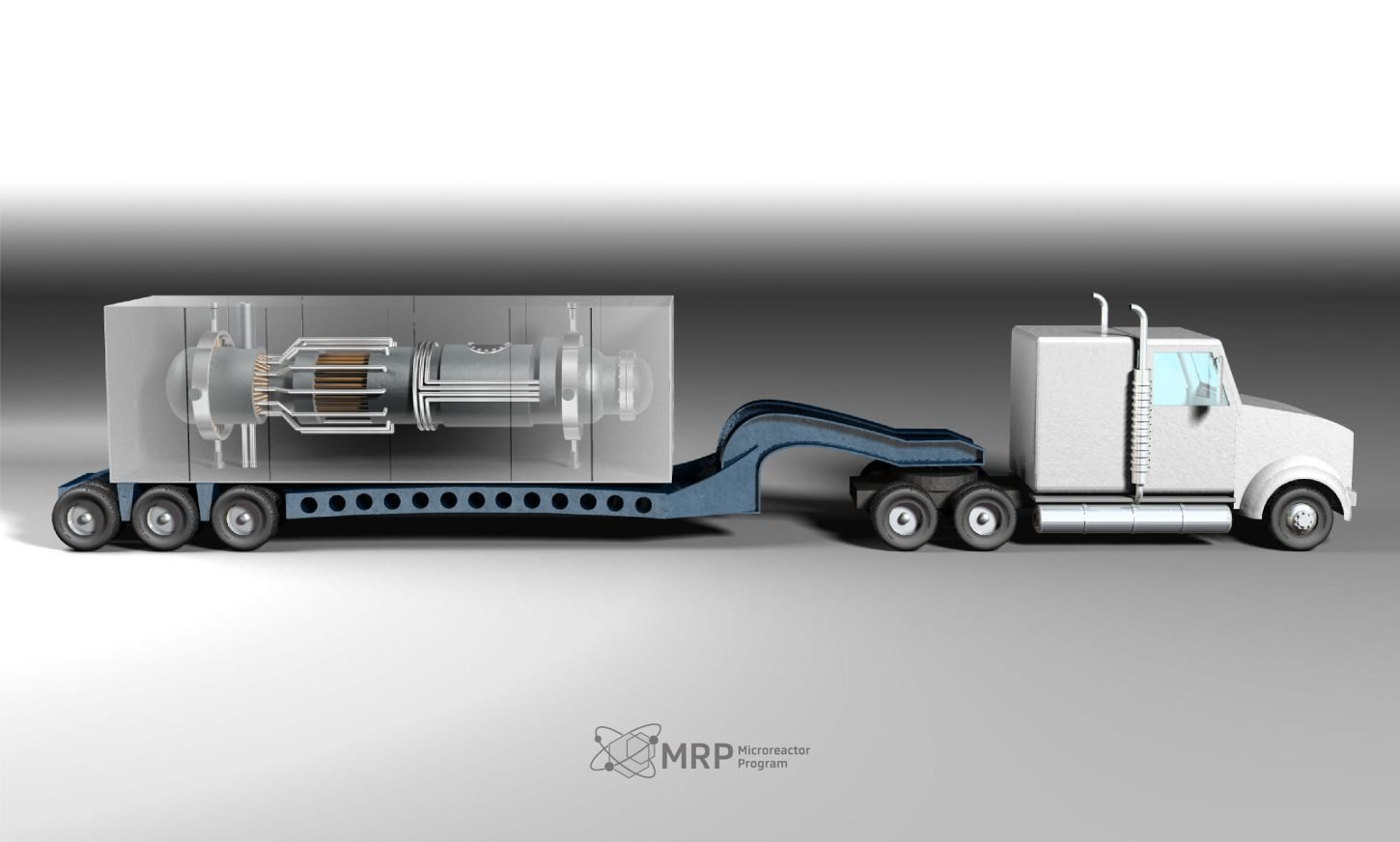
Image courtesy of INL.gov, showcasing a microreactor being transported.
Key Features
- ✓ Size and Portability: 100 to 1,000 times smaller than traditional reactors, factory-built, and transportable by semi-truck.
- ✓ Fuel and Operation: Uses low-enriched uranium for years of operation without refueling; some use sodium-potassium coolant for natural circulation.
- ✓ Applications: Reliable, carbon-free energy for remote communities, military operations, industrial processes, data centers, and space propulsion.
- ✓ Safety and Regulation: Enhanced safety through advanced materials, sensors, and simplified designs, with modernized licensing support.
Physical Description (Based on INL's MARVEL Microreactor)
The MARVEL (Microreactor Applications Research Validation and Evaluation) microreactor at Idaho National Laboratory (INL) serves as a concrete example:
- ● Structure: Full-scale, electrically heated stainless-steel apparatus with a sleek, cylindrical design, including a main vessel body and intermediate heat exchangers.
- ● Components: Features a primary coolant system (sodium-potassium cooled), guard vessel, reactor support frame, and reactivity control system.
- ● Size: Designed to fit within INL's facilities, generating 85-100 kilowatts of thermal energy (convertible to ~20 kilowatts of electricity), underscoring its compact scale.
Lunar Resource Exploration
The future of energy and critical minerals extends beyond Earth. Lunar resource exploration is crucial for establishing long-term human presence in space and securing vital materials.
Conceptual image generated by AI, illustrating lunar resource extraction or base.
Non-Traditional Materials in Development at Idaho National Labs
INL is at the forefront of developing advanced materials crucial for next-generation nuclear reactors, enabling higher efficiencies, enhanced safety, and diverse applications beyond traditional designs.
1. TRISO (Tri-structural Isotropic) Fuel
AI-generated visual of TRISO fuel particles.
Uranium-based fuel kernels coated with multiple layers of carbon and silicon carbide. Highly durable and accident-tolerant, designed for high-temperature gas-cooled reactors and microreactors.
- • Withstands temperatures up to $1,800^{\circ}C$.
- • Enhanced safety by containing fission products.
2. Molten Salt Fuel and Components
AI-generated visual of molten salt fuel within a reactor.
Molten salt reactors (MSRs) use liquid fuel (chloride or fluoride salts) as both fuel and coolant. INL develops corrosion-resistant materials for components.
- • Higher thermal efficiency ($600-800^{\circ}C$ operating temp).
- • Reduced risk of meltdown.
3. Advanced Structural Materials
Researching high-temperature materials like molybdenum, tungsten, or silicon carbide-based ceramics for reactor components.
- • Operates above $1,000^{\circ}C$.
- • Greater resistance to radiation damage and corrosion.
4. Heat Pipe Materials
Used in heat pipe-cooled microreactors, these systems use liquid metal (sodium or potassium) in sealed pipes to transfer heat passively.
- • Passive cooling enhances safety.
- • Compact design ideal for remote/space applications.
5. Accident-Tolerant Fuels (ATF) and Cladding
Exploring non-traditional cladding materials like silicon carbide or iron-chromium-aluminum (FeCrAl) alloys to improve safety during accidents.
- • Higher oxidation resistance in high-temperature steam.
- • Improved mechanical strength and radiation tolerance.
Methods for H2S Removal as a Liquid
Hydrogen sulfide (H2S) in agricultural silos is a significant gaseous contaminant. Liquid-based removal methods are crucial for safe extraction and preventing degradation of advanced materials.
1. Chemical Absorption with Alkaline Solutions
H2S is absorbed into solutions like sodium hydroxide (NaOH) or calcium hydroxide (Ca(OH)2) to form non-volatile compounds. High removal efficiency ($>99\%$).
Example: H2S + 2NaOH → Na2S + 2H2O
2. Ionic Liquids (ILs) for Selective H2S Capture
Reusable solvents (e.g., triethylenetetramine-based ILs) absorb H2S selectively over CO2 or CH4. Low vapor pressure and high thermal stability.
3. Aqueous Amine Scrubbing (Alkanolamines)
Solutions like MEA, DEA, or MDEA absorb H2S via reversible chemical reactions. High removal efficiency ($98-99\%$) and regenerable.
Example: H2S + R-NH₂ → R-NH₃⁺HS⁻
4. Chemical Oxidation in Liquid Phase
H2S is oxidized in a liquid medium using agents like hydrogen peroxide (H₂O₂). Effective for preventing H2S exposure to sensitive materials.
Specific INL Projects and Timeline
Idaho National Lab is actively involved in several key projects advancing nuclear energy technologies, with significant milestones expected in the coming years.
Antares Nuclear Microreactor: A 300-kW heat pipe-cooled design under development for testing in DOME.
MARVEL Microreactor: A DOE project to demonstrate a 100-kW microreactor using advanced fuels and heat pipe cooling, with INL leading material testing.